Bringing innovation to intestinal parasite treatment
An international partnership to implement a new two-in-one medicine for helminthiasis

An innovative dual approach supported by evidence and regulators
Approaching the finish line for our novel single-tablet treatment
The STOP2030 research project works on providing a single tablet that combines two proven medicines to more effectively treat soil-transmitted helminthiasis, a disease caused by parasitic intestinal worms.
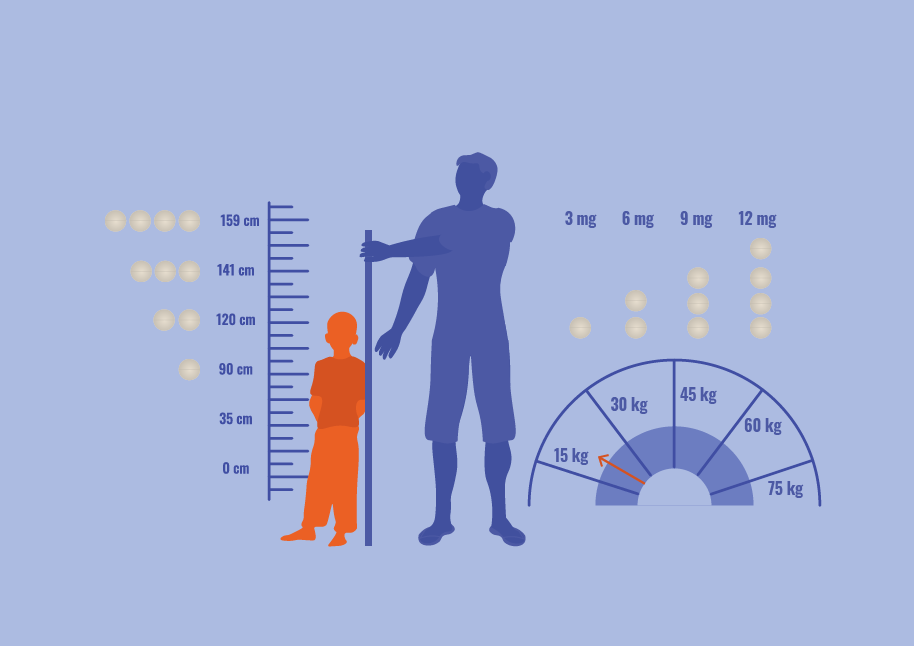
The STOP2030 project aims to advance the widespread adoption of a fixed-dose, orodispersible tablet that combines ivermectin and albendazole. This coformulation targets all the soil-transmitted helminth (STH) species considered by WHO, provides superior efficacy, comparable safety and logistic simplicity.
In the last few years, our innovative tablet’s safety and efficacy have been evaluated in a pivotal clinical trial in three countries (STOP I AND II), and has received regulatory support. Building from these milestones, STOP2030 aims to complete the clinical and implementation evaluations and provide a new, better tool to achieve global control goals for Neglected Tropical Diseases.
Proven safety and superior efficacy
Explore the results of our phase 2/3 clinical trial, where we evaluated our co-formulation against albenzadole in over 1,000 participants in Kenya, Ethiopia and Mozambique.
With a favourable evaluation by EMA
Our co-formulation received a European Medicines Agency (EMA)’s positive scientific evaluation for treatment of soil-transmitted helminthiases and lymphatic filariasis.
The making of our
co-formulation
Building upon 10+ years of research
and collaboration
Our search for a more effective treatment for soil-transmitted helminth (STH) infections started in the early 2010s in Northern Argentina, driven by the need to address existing treatment gaps. That started a journey of collaboration between leading institutions from Africa, Latin America, North America and Europe to carry out extensive research and clinical trials. We believe the scientific output that supports the fixed-dose coformulation of albendazole and ivermectin speaks volumes.


Tailored to help address a global health problem
A new tool to achieve the WHO’s 2030
Roadmap goals
WHO estimates that 1.5 billion people are infected with soil-transmitted helminths, primarily in areas with poor sanitation. In these regions, worm eggs are excreted in faeces and can be accidentally ingested, continuing the cycle of infection. Control efforts mainly rely on mass drug administration (MDA) campaigns, which have helped reduce the burden but often fall short of achieving control targets. Challenges such as drug resistance, limited treatment coverage, and logistical difficulties highlight the urgent need for a more effective alternative.
Translating research into action
A global effort towards implementation
To effectively tackle the problem caused by intestinal worms, it is essential to co-create solutions with countries affected by the disease. Public-private partnerships have proven their effectiveness in achieving progress. Our team is built by partners from Kenya and Ghana research and public health sectors, as well as international public health organizations and a private sector pharmaceutical group. The collaboration between continents and areas of expertise helps identify and overcome challenges that may limit implementation, accelerating the future treatment’s availability for all those who need it.